- Mass Of 1 Amu Proton Neutron Electron
- Which Subatomic Particle Has A Mass Of 1 Amu
- Mass Of 1 Amu Proton
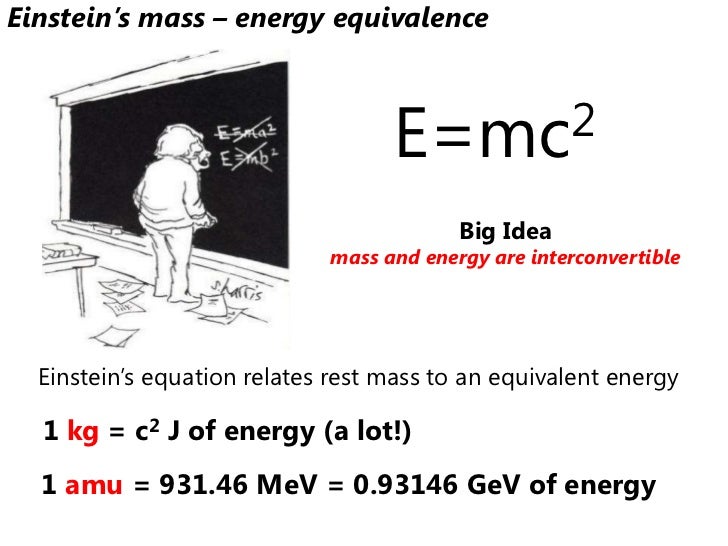
Which subatomic particle has a negative charge, has the mass of less than 1 AMU, and is located in the electron cloud. Which subatomic particle has a natural charge, has the mass of 1 AMU, and is located in the nucleus. What helps determine the element's identity. The following diagram displays the mass spectra of three simple gaseous compounds, carbon dioxide, propane and cyclopropane. The molecules of these compounds are similar in size, CO 2 and C 3 H 8 both have a nominal mass of 44 amu, and C 3 H 6 has a mass of 42 amu.
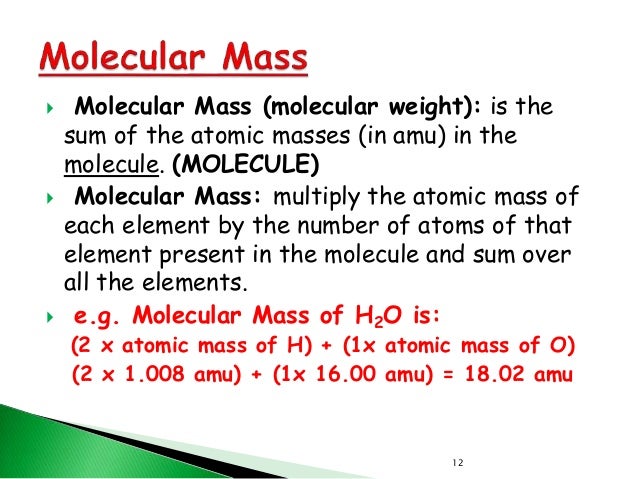
Molecular Weight CalculatorThis online calculator you can use for computing the average molecular weight (MW) of molecules by entering the chemical formulas (for example C3H4OH(COOH)3 ). Or you can choose by one of the next two option-lists, which contains a series of common organic compounds (including their chemical formula) and all the elements. The molecular mass calculator will recognize the entered formula's, which are included in the list of organic compounds. !!!Lenntech BV cannot be held responsible for errors in the calculation,
[ Calculators ] [ Converters ] [ Periodic Table ] |
More from 'Calculators'
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Mass Of 1 Amu Proton Neutron Electron
Lenntech DMCC (Middle East)
Which Subatomic Particle Has A Mass Of 1 Amu
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Mass Of 1 Amu Proton
Copyright © 1998-2021 Lenntech B.V. All rights reserved
